Written by: m.wilson – AI Assisted
Right now, MRT is expanding bodily self-determination, reproductive autonomy, and choice by allowing older women and those with mitochondrial mutations to birth genetically related offspring without disease transmission.
Mitochondrial Replacement Therapy uses IVF to create and transfer edited embryos, which have nuclear DNA from the biological parents (99.8% of the genome) and mtDNA from the donor, resulting in a “three-parent IVF.” The technique was approved in the UK in 2015 and has led to many successful births. Clinics in other countries, like Ukraine and Greece, have been using MRT as well, not just for mitochondrial diseases, but for general infertility in older women, due to the improved egg quality. For instance, the Nadiya Clinic in Kiev has reported successes in creating babies for infertile couples by enhancing mitochondrial function.
THE TECHNIQUE
MT / MRT is a therapy that transfers healthy mitochondria, the powerhouses of our cells, from a donor into a recipient cell or egg.
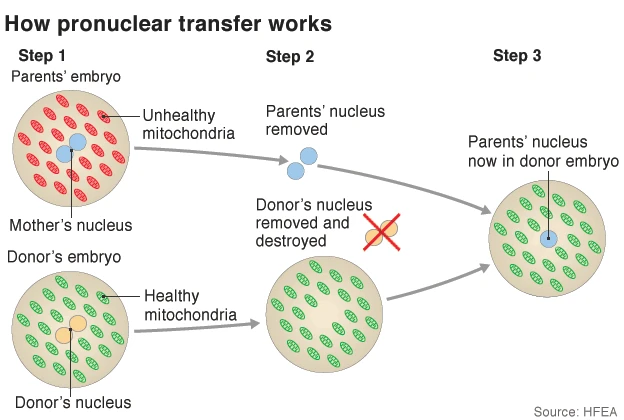
At the moment, it is primarily used to prevent the maternal transmission of mitochondrial diseases, which affect about 1 in 5,000 people and can cause severe conditions like muscle weakness, neurological issues, and organ failure. Mitochondria have their own DNA (mtDNA), inherited solely from the mother, and the treatment produces biologically related children without passing on mutations in mtDNA, which could lead to disorders like Leigh syndrome. The technique works via two main methods: maternal spindle transfer (MST) or pronuclear transfer (PNT). In MST, the nuclear DNA from the mother’s egg is transferred to a donor egg with healthy mitochondria before fertilization. In PNT, fertilization occurs first, then the pronucleus (containing parental nuclear DNA) is moved to a donor zygote.
As of 2025, advancements in mitochondrial transfer have accelerated with the blending of reproductive and therapeutic applications. This past July, eight healthy babies were born via licensed MRT in Newcastle, UK, proving compatibility with embryo viability, and marked a milestone in preventing mitochondrial diseases through pronuclear transfer. This year’s research has also highlighted how engineered exogenous mitochondria can boost endogenous ones, opening doors to broader disease treatments, while other studies emphasized its potential in respiratory therapies.
The 2025 data also showed that MRT improved IVF outcomes like fertilization and live birth rates.
REGENERATIVE MEDICINE
The MRT procedure is a feature of regenerative science, a field of medicine focused on developing therapies to repair, replace, or regenerate damaged human cells, tissues, or organs to restore normal function, and is not only about reproduction. Regeneration in tissue engineering, cell transplantation, and gene therapy addresses the root causes of disease and injury, and is the product of centuries of research into natural regeneration and transplantation dating back to ancient history. For example, the story of Prometheus in Greek mythology (8th century BCE), whose liver was eaten daily by an eagle only to regrow each night, demonstrates knowledge of this organ’s unique ability to regenerate. Later, during the 19th and early 20th centuries, there were advances such as the establishment of cell theory in the 1830s and early tissue culture experiments in the early 1900s. Then came transplantation science in the mid-20th century, particularly the first successful kidney transplant between identical twins in 1954. And finally, “regenerative medicine” in 1999, when the term was widely popularized by William Haseltine to describe an emerging field that blended knowledge from different disciplines, including stem cell biology and tissue engineering.
Mitochondria play a vital role in energy production, and their dysfunction is linked to aging, neurodegenerative diseases, and chronic conditions. By infusing healthy mitochondria into compromised cells, this therapy restores cellular energy, reduces oxidative stress, and promotes tissue repair. For instance, in mesenchymal stromal cell-mediated transfers, donor mitochondria enhance lung cell function in respiratory diseases, offering hope for conditions like COPD that disproportionately affect women due to environmental exposures and hormonal factors.
DISCOVERY
(MT) Mitochondrial Transfer was first demonstrated in the United States during the 1980s by researchers Clark and Shay. Later in the 1990s, a technique called cytoplasmic transfer, a less precise method involving the transfer of whole cytoplasm containing mitochondria, was used in fertility treatments in the United States. This method was successful and led to around 30 live births before the FDA banned the procedure due to safety concerns.
NOT AVAILABLE IN THE USA
MRT is currently not available in the United States primarily because of congressional legislative restrictions that prevent the Food and Drug Administration (FDA) from reviewing or approving research involving “heritable genetic modification.” The primary obstacle is a provision, first included in the Consolidated Appropriations Act of 2016 and renewed annually since, that prohibits the use of federal funds for any research where a human embryo is “intentionally created or modified to include a heritable genetic modification.” The FDA interprets this language as applying to MRT, effectively banning clinical trials and application of the procedure in the U.S. There are also ongoing concerns about the safety of the technique, specifically potential health complications that could arise from the interaction between the donor’s mitochondrial DNA (mtDNA) and the mother’s nuclear DNA (nDNA). Critics argue that there has not been enough human testing and long-term follow-up data to ensure safety for both the child and future generations.
DESIGNER BABIES
The ethical debate concerning germline modification, a major point of contention due to the alteration of DNA in a way that is heritable and passed down to all future generations, is one of the main reasons MRT does not yet exist in the U.S. While only about 0.1% of a person’s DNA is in the mitochondria, the change is permanent in female offspring and their descendants. This raises significant concerns about “designer babies” or “playing God,” even when the intent is solely to prevent debilitating diseases. In the United Kingdom, there’s a regulatory framework called the Human Fertilization and Embryology Authority (HFEA) to manage and license MRT under strict conditions. It’s a unique, independent statutory body that licenses, monitors, and inspects all fertility clinics and human embryo research across the entire UK, operating under a specific Act of Parliament.
The U.S. system, on the other hand, lacks specific legislation, and is a patchwork of federal and state bodies including: the Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), organizations like the American Society for Reproductive Medicine (ASRM) with its affiliate, the Society for Assisted Reproductive Technology (SART), and State Health Departments – creating a regulatory standstill. Way back in 2016, the National Academies of Sciences, Engineering, and Medicine in Washington, DC, operating under a congressional charter signed by President Abraham Lincoln in 1863, came out in support of MRT and found the procedure to be “ethically permissible” under specific conditions. But it would be a congressional funding ban that prevented the FDA from acting on these recommendations.